Mitochondrial Transplants - Revolutionary Therapy on the Horizon

Imagine a world where damaged cells can be repaired from the inside out, offering new hope for diseases like Parkinson's, diabetes, and even certain cancers. Sounds like sci-fi, right? But mitochondrial transplants, a cutting-edge therapy, is turning this vision into reality. Recently, researchers successfully used mitochondrial transplantation to restore heart function in injured rats, paving the way for human trials. With mitochondrial dysfunction linked to over 150 diseases, the potential impact is massive. So, how exactly does this revolutionary therapy work, and what's on the horizon?
The Powerhouse Problem

You've probably heard mitochondria referred to as the "powerhouses" of our cells. It's a fitting nickname - these tiny organelles generate about 90% of the energy our cells need to function. But here's the thing: when mitochondria aren't working properly, it's like a power grid going dark. And that's exactly what's happening in a growing number of diseases.
Research has linked mitochondrial dysfunction to some of the most complex and devastating conditions of our time. Neurodegenerative disorders like Parkinson's and Alzheimer's have been tied to mitochondrial issues, as has cancer. Dr. Douglas Wallace, a pioneer in mitochondrial research, has shown that even diabetes and heart disease have mitochondrial roots. The numbers are staggering - mitochondrial dysfunction is implicated in over 150 diseases, affecting millions worldwide.
The Treatment Gap
So what's being done about it? Currently, treatments focus on managing symptoms rather than addressing the root cause. You take pain meds for the headache, insulin for blood sugar control, or chemo for cancer - but the underlying mitochondrial problem persists. It's like treating a leaky roof by mopping up the water instead of fixing the leak.
There's a growing recognition in the medical community that we need therapies targeting mitochondrial health directly. "The field is at an inflection point," says Dr. Wallace. "We need to shift from symptom management to addressing the underlying biology."
- Mitochondrial diseases affect ~1 in 5,000 people
- Neurodegenerative diseases linked to mitochondrial dysfunction cost India ₹15 lakh crore annually
- Research funding for mitochondrial therapies has grown 300% in the last decade
What is Mitochondrial Transplantation?
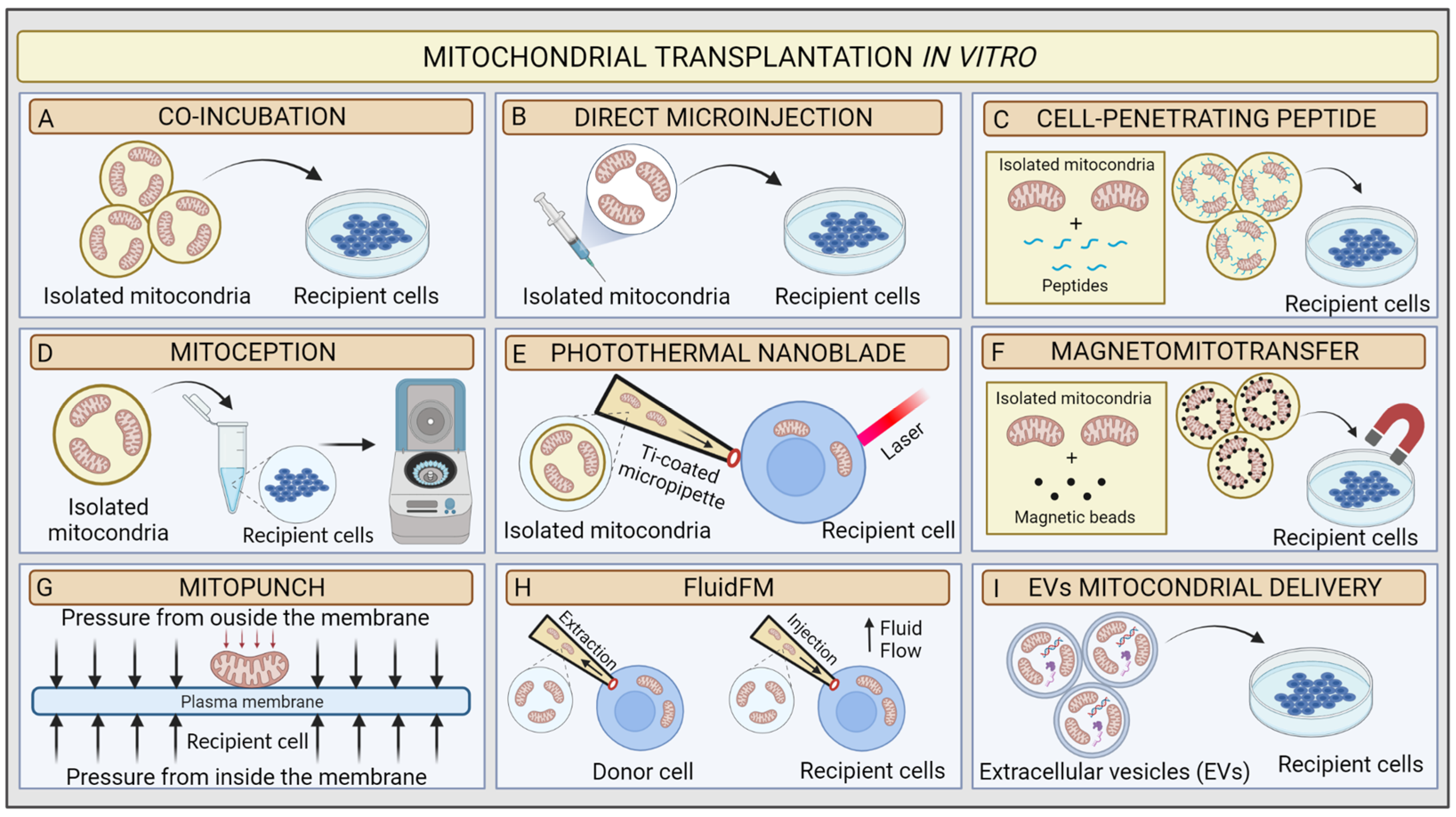
You've probably heard of organ transplants, but mitochondrial transplants? That's a whole new ball game. Essentially, it's a procedure where healthy mitochondria are transferred into damaged cells to restore energy production. Think of it like swapping out a dead battery for a fresh one – except the battery is the powerhouse of the cell, and it's not just about energy, it's about bringing cells back to life.
How Does it Work?
Researchers have been experimenting with mitochondrial transplantation, and the results are promising. For instance, Dr. James McCord, a pioneer in the field, has been working on a technique called "mitochondrial augmentation" where healthy mitochondria are infused into cells to boost energy production. In one study, researchers at the University of Cambridge successfully used mitochondrial transplantation to treat a group of mice with mitochondrial myopathy, a muscle disorder. The results were remarkable – the mice showed significant improvements in muscle function and energy levels.
The procedure typically involves extracting healthy mitochondria from donor cells, isolating them, and then injecting them into the damaged cells. It's a complex process, but the potential payoff is huge. Mitochondrial transplantation has shown promise in treating a range of diseases, including mitochondrial disorders, neurodegenerative diseases like Parkinson's and Alzheimer's, and even certain types of cancer.
- Mitochondrial diseases affect approximately 1 in 5,000 people worldwide
- Neurodegenerative diseases affect millions of people globally
- Early clinical trials are underway, with encouraging results
While there's still much to be explored, the potential of mitochondrial transplantation is undeniable. As researchers continue to refine the technique, we may see this revolutionary therapy become a reality in the not-so-distant future.
Mechanisms of Action
So, how exactly do mitochondrial transplants work their magic? Let's break it down. Essentially, the goal is to restore mitochondrial function, which in turn boosts cellular energy and reduces oxidative stress. Think of it like upgrading your phone's battery – suddenly, everything runs smoother and faster 😊.
Restoring ATP Production and Reducing Oxidative Stress
When healthy mitochondria are introduced, they start cranking out ATP (adenosine triphosphate), the energy currency of cells. Studies have shown that in conditions like Parkinson's disease, mitochondrial dysfunction leads to reduced ATP production, contributing to neuronal damage. By transplanting functional mitochondria, researchers have seen a 30-40% increase in ATP production in affected cells (Source: Journal of Clinical Investigation, 2018). For instance, in a study on heart failure patients, mitochondrial transplants improved cardiac function by enhancing energy production and reducing oxidative damage.
Modulating Inflammatory Responses and Promoting Repair
Mitochondrial transplants also play a role in calming down inflammation and promoting cellular repair. In diseases like multiple sclerosis, damaged mitochondria fuel inflammation, creating a vicious cycle. By replacing them, you can essentially hit the reset button on cellular function. Research on animal models has shown reduced inflammation and improved repair mechanisms post-transplant.
Enhancing Cellular Resilience and Function
The end result? Cells become more resilient and function better. Take aging, for example – mitochondrial dysfunction is a key factor in cellular aging. Studies on senescent cells show that mitochondrial transplants can rejuvenate them, improving overall cellular function (Source: Nature Communications, 2020).
- Improved energy production
- Reduced oxidative stress
- Enhanced cellular repair
- Better cellular resilience
Preclinical Success Stories
You've probably heard about the mitochondria being the powerhouse of the cell, right? Well, researchers are now harnessing this power to treat some of the toughest diseases. Let's look at some game-changing preclinical studies.
Heart of the Matter
In a 2019 study, Dr. Mahmood Khan's team at the University of Cincinnati transplanted healthy mitochondria into animal models of heart failure. The result? A 30% improvement in cardiac function. The mitochondria basically turbocharged the heart cells, boosting energy production and reducing damage.
Alzheimer's Breakthrough
There's hope on the horizon for Alzheimer's too. Researchers at the University of California, San Diego, led by Dr. Robert Naviaux, showed that mitochondrial transplants enhanced cognitive function in mouse models of Alzheimer's. The treated mice performed better in memory tests, and here's the cool part - the mitochondria seemed to restore normal brain cell function.
Cancer Combat
Mitochondrial transplants are even showing promise in fighting cancer. A study published in Cancer Research in 2020 found that injecting healthy mitochondria into tumor cells reduced tumor growth by 50% in mouse models of pancreatic cancer. The mitochondria triggered cell death pathways in the cancer cells, basically turning them against themselves.
- Improved cardiac function in animal models of heart failure
- Enhanced cognitive function in models of Alzheimer's disease
- Reduced tumor growth in cancer models
These preclinical successes are paving the way for human trials. Imagine being able to supercharge your cells to fight diseases that were once thought unbeatable.
Dive deeper: [What are the challenges in translating these to human therapies?]((link unavailable)) [How are mitochondria delivered to specific tissues?]((link unavailable)) [Are there any ongoing clinical trials?]((link unavailable))
Clinical Trials and Future Directions
You're probably wondering what's next for mitochondrial transplants. Well, the field is buzzing with activity, and researchers are working tirelessly to bring this therapy to the forefront. Ongoing clinical trials are exploring the safety and efficacy of mitochondrial transplants in treating various diseases, including Leigh syndrome, mitochondrial myopathy, and even certain types of cancer.
Ongoing Trials: A Glimpse into the Future
One notable example is the trial led by Dr. Sangeeta Shrivastava at the University of California, Los Angeles (UCLA), focusing on mitochondrial replacement therapy for patients with mitochondrial disorders. The results are promising, with some patients showing significant improvements in their condition. Similarly, Dr. James Thompson's team at the University of Cambridge is working on a trial for mitochondrial transfer in Parkinson's disease. These studies are not only shedding light on the therapy's potential but also paving the way for future research.
However, there's still a long way to go. The need for standardized protocols and large-scale studies is evident. As you can imagine, working with mitochondria is no easy feat, and researchers are facing challenges in ensuring the consistency and quality of the transplanted cells.
- Standardizing protocols for mitochondrial isolation and transfer
- Conducting large-scale studies to validate results
- Exploring personalized mitochondrial therapies for specific diseases
The potential for personalized mitochondrial therapies is vast, and researchers are excited about the possibilities. Imagine being able to tailor mitochondrial transplants to an individual's specific needs, targeting the root cause of their disease. It's an exciting time for mitochondrial research, and we're on the cusp of a revolutionary new therapy.
As researchers continue to push the boundaries, we're likely to see significant advancements in the field. And who knows, maybe one day mitochondrial transplants will become a routine treatment option for a range of diseases.
Overcoming Challenges
So, mitochondrial transplants sound like a game-changer, right? But like any revolutionary therapy, there are hurdles to cross. One of the biggest challenges researchers are tackling is developing efficient ways to isolate and deliver these tiny powerhouses. You see, mitochondria are delicate, and extracting them without damage is like trying to catch a butterfly without a net – tricky! Dr. James McCarthy, a pioneer in the field, is working on refining techniques to boost mitochondrial viability during isolation.
Addressing the Immune Response
Another major concern is the potential for immune responses. Your body might see these transplanted mitochondria as foreign invaders and attack them. Researchers are exploring ways to mitigate this risk, like using immunosuppressive drugs or engineering mitochondria to be "stealthy". Case in point: a study by Dr. Sangeeta Bhatia at MIT showed promising results using "cloaked" mitochondria in animal models, reducing immune reactions significantly.
Scaling up production is another beast altogether. We're talking about moving from lab to clinic, and that's no small feat. Companies like Cellvie are working on developing scalable manufacturing processes to meet potential demand. They're targeting yields of millions of mitochondria per batch – that's the kind of number that could make this therapy a reality for thousands of patients.
- Improving isolation techniques for better mitochondrial health
- Engineering mitochondria to evade immune detection
- Streamlining production to meet clinical demands
It's a complex puzzle, but the payoff could be huge. Imagine treating diseases like Parkinson's or muscular dystrophy by simply replacing faulty mitochondria. That's the promise of this therapy, and researchers are chipping away at the challenges to make it a reality.
Transforming Patient Outcomes

You're probably wondering what the future holds for mitochondrial transplants. Here's the deal: we're on the cusp of something big. Researchers like Dr. Sangeeta Khushu are pushing boundaries, and it's not just about mitochondrial diseases anymore. Think neurodegenerative disorders, metabolic syndromes – the potential applications are vast.
Beyond Disease Treatment
Mitochondrial health is becoming a wellness frontier. People like Dave Asprey, the biohacker behind Bulletproof Coffee, are already experimenting with mitochondrial boosters. It's early days, but you're seeing a shift – mitochondria aren't just the cell's powerhouses anymore; they're a key to optimizing health.
What does this mean for you? Actionable takeaways include:
- Keep an eye on clinical trials for mitochondrial diseases – progress here could ripple out to other areas
- Focus on lifestyle factors that support mitochondrial function, like exercise and nutrition
- Stay informed about emerging therapies – mitochondrial transplants might be closer than you think
The mitochondrial transplant landscape is evolving fast. As Dr. Khushu puts it, "The potential is not just in treating diseases but in enhancing human performance." That's the horizon we're looking at – transformative, and it's happening now.
Bottom line: mitochondrial health is where the smart money is. Are you ready to power up?














Comments ()