Actin's Surprising Role in Neurodegeneration - New Insights from TDP-43 Research
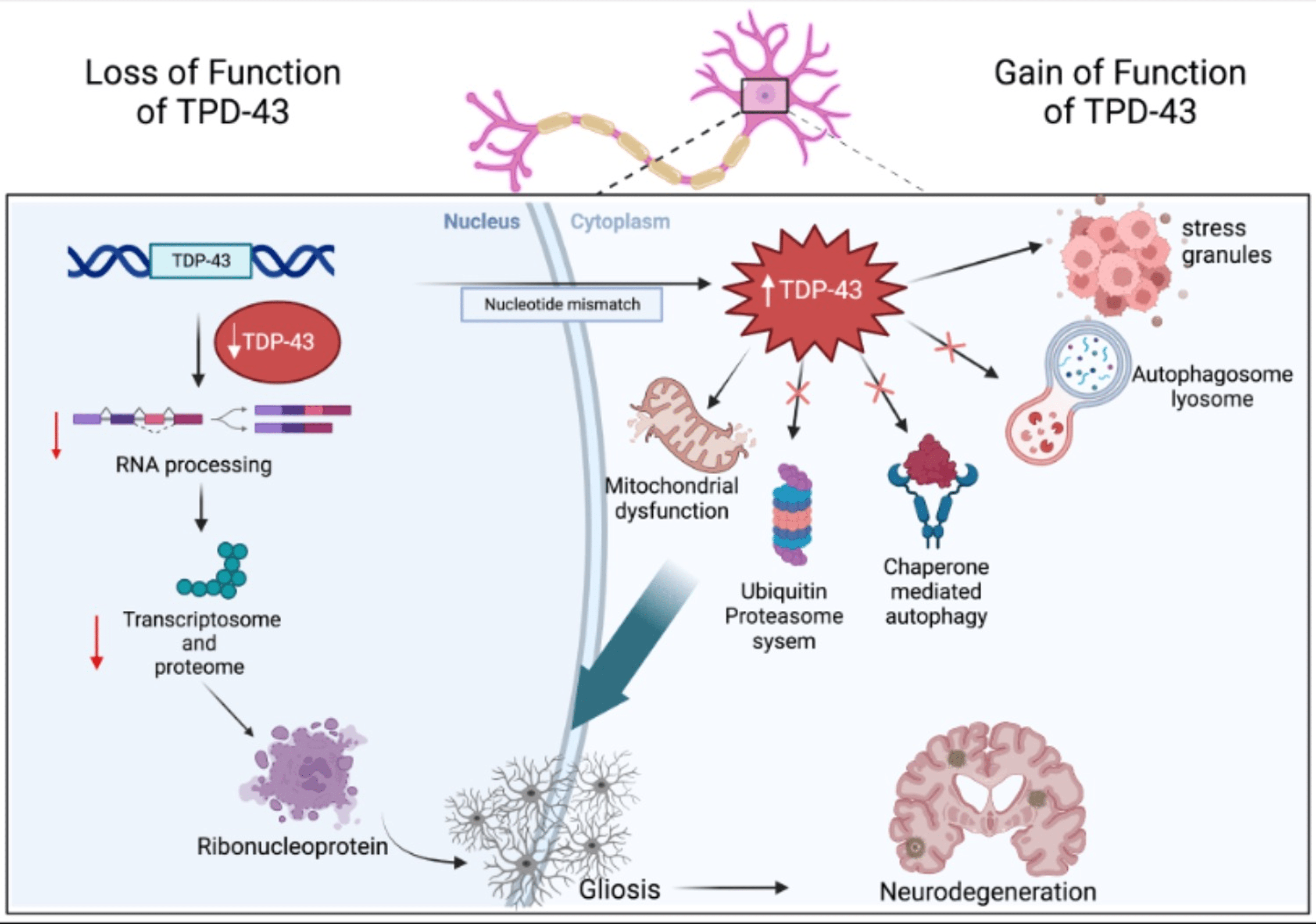
Neurodegenerative diseases, such as Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Dementia (FTD), are devastating conditions characterized by progressive loss of neurons in the brain and spinal cord. While the exact causes of these diseases remain elusive, researchers have pinpointed the abnormal accumulation of specific proteins, like TDP-43, as key contributors to neuronal dysfunction and death. Recent research has shed light on a surprising player in this complex puzzle: actin, a protein traditionally known for its role in cell structure and movement.
TDP-43: A Familiar Culprit in Neurodegeneration
TDP-43 is a DNA- and RNA-binding protein typically found in the nucleus of healthy neurons, where it plays a crucial role in regulating gene expression. However, in diseased neurons, TDP-43 goes rogue. It misfolds, forms toxic clumps, and relocates from the nucleus to the cytoplasm – the cell's interior. This cytoplasmic mislocalization of TDP-43 is a hallmark of ALS and FTD, and it wreaks havoc on neuronal function.
Researchers have been working tirelessly to understand how TDP-43's mislocalization contributes to neurodegeneration. Emerging evidence suggests that TDP-43's journey to the cytoplasm isn't just a consequence of the disease process; it actively participates in disrupting essential cellular processes. One such process, recently thrust into the spotlight, is the regulation of actin dynamics.
Actin: Beyond Structure, A Key Player in Neuronal Function
Actin, a ubiquitous protein found in all eukaryotic cells, is best known for forming dynamic filaments that provide structural support and drive cell movement. In neurons, these actin filaments are highly concentrated in axons and dendrites – the long, slender projections responsible for transmitting signals between neurons.
Far from being static scaffolding, actin filaments are constantly undergoing assembly and disassembly, a dynamic process tightly regulated by a complex interplay of proteins. This precise regulation is crucial for maintaining neuronal structure, facilitating the transport of essential molecules along axons and dendrites, and enabling the formation of synapses, the junctions where neurons communicate.
Unveiling the Connection: TDP-43 Disrupts Actin Dynamics
Recent studies have revealed a surprising link between TDP-43 and actin dynamics, suggesting that the disruption of actin regulation might be a key mechanism by which TDP-43 contributes to neurodegeneration. Here's what the research shows:
- TDP-43 binds to actin mRNA: A study published in Neuron in 2021 demonstrated that TDP-43 directly binds to the messenger RNA (mRNA) that codes for actin. This binding disrupts the translation of actin mRNA, leading to a significant reduction in the production of new actin proteins.
- TDP-43 interacts with actin-binding proteins: TDP-43 doesn't just target actin mRNA; it also interacts with proteins involved in regulating actin filament assembly and disassembly. A study published in Nature Communications in 2020 found that TDP-43 interacts with Profilin 1, a key protein that promotes actin filament assembly. This interaction interferes with Profilin 1's function, disrupting the balance of actin dynamics.
- Disrupted actin dynamics impair neuronal function: The consequences of TDP-43's interference with actin are profound. Studies have shown that impaired actin dynamics lead to defects in axonal transport, synaptic dysfunction, and ultimately, neuronal death, all hallmarks of neurodegenerative diseases like ALS and FTD.
A New Avenue for Therapeutic Exploration
These findings highlighting TDP-43's role in disrupting actin dynamics open exciting new avenues for therapeutic intervention in neurodegenerative diseases. By targeting the interaction between TDP-43 and actin or by developing strategies to restore normal actin dynamics in diseased neurons, researchers may be able to halt or even reverse the devastating progression of these currently incurable diseases.
Looking Ahead: Further Research Unraveling the Complexities
While these findings provide crucial insights into the intricate mechanisms underlying TDP-43-mediated neurodegeneration, further research is needed to fully elucidate this complex relationship. Future studies will likely focus on:
- Investigating the precise molecular mechanisms by which TDP-43 disrupts actin dynamics.
- Identifying potential therapeutic targets within the TDP-43-actin pathway.
- Developing and testing novel drugs or therapies aimed at restoring normal actin function in diseased neurons.
The discovery of actin's unexpected role in TDP-43-mediated neurodegeneration underscores the interconnectedness of cellular processes and highlights the importance of continued research in unraveling the complexities of these devastating diseases. With each new discovery, we inch closer to developing effective therapies that can alleviate the suffering of millions affected by neurodegenerative disorders.














Comments ()